In an effort to curb climate change, targets to reduce greenhouse gas emissions are imposed by governments around the work. Mineral, chemical, refining, iron and steel industries contribute significantly to the total anthropogenic CO2 emissions. Strategies to achieve emissions reductions include green electricity, electrifying heating and industrial plasmas. Plasmas have a key role to play in this.
The nonequilibrium distribution of energy in nonthermal plasma provides unique opportunities for energy and gas conversion. Molecules that require high temperature and/or pressure to activate by thermochemical processes, like CH4, CO2, or N2, can be activated in nonthermal plasma by excitation or dissociation through collisions with high energy electrons. As a result, nonthermal plasma offers an electrically driven, near-ambient temperature and pressure method for chemical conversion for energy storage applications.
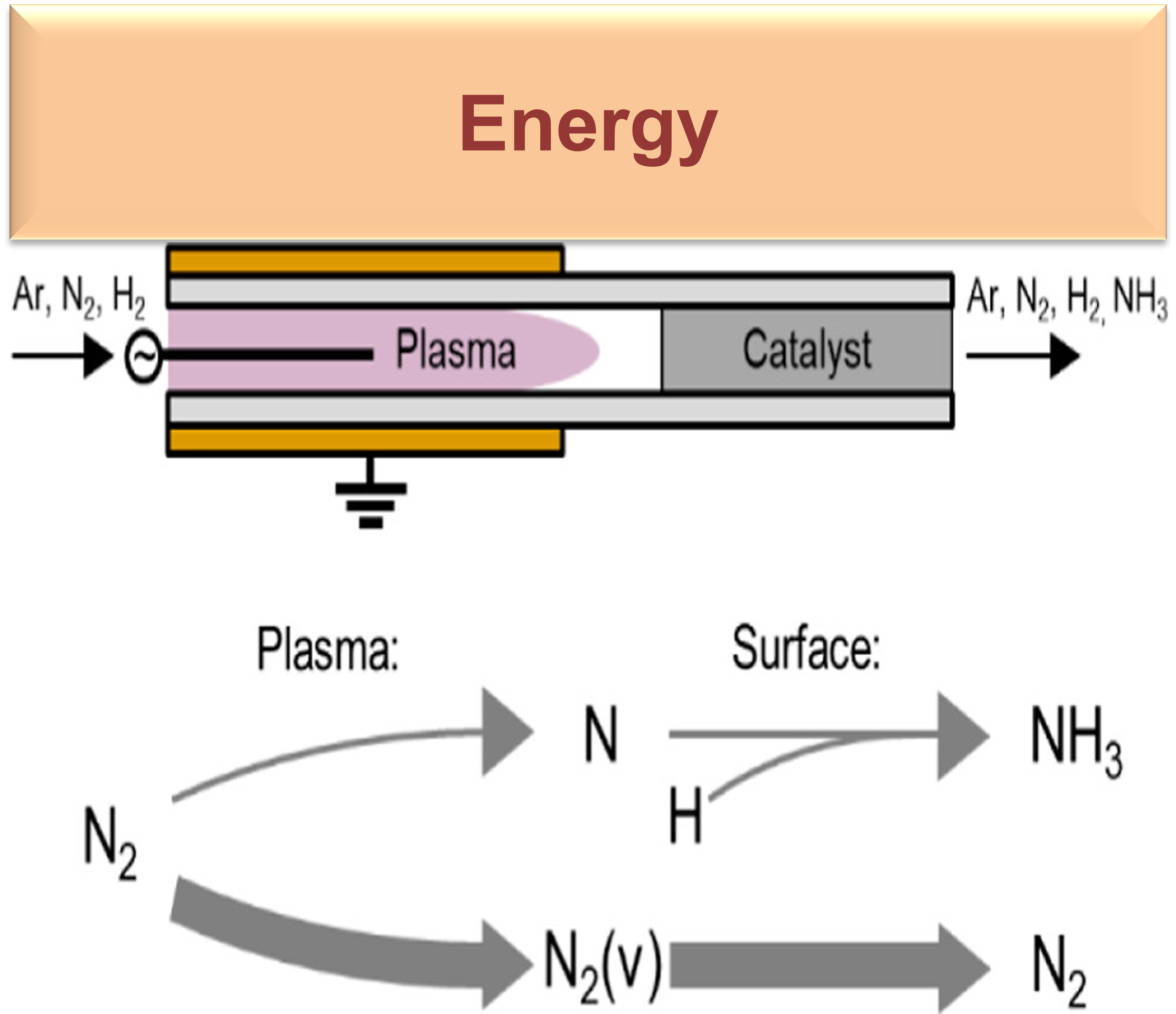
Work in our group has focused on elucidating pathways for plasma-driven production of CH3OH or NH3, two potential molecules for energy storage, from CH4 and O2 or N2 and H2, respectively. These transformations occur through a series of physical and chemical processes that occur in nonthermal plasma and (possibly) on catalytic surfaces. Understanding the species and reactions that drive chemical conversion is enabled by use of molecular beam mass spectrometry or various optical diagnostic techniques available in our lab.
Further readings:
J. Jiang and P. Bruggeman. “Investigation of the Mechanisms Underpinning Plasma-Catalyst Interaction for the Conversion of Methane to Oxygenates” Plasma Chemistry and Plasma Processing (2022) 42:689–707 https://doi.org/10.1007/s11090-022-10251-5
B. Bayer, P. Bruggeman, and A. Bhan. “Species, Pathways, and Timescales for NH3 Formation by Low-Temperature Atmospheric Pressure Plasma Catalysis” ACS Catalysis (2023) 13, XXX, 2619–2630 https://doi.org/10.1021/acscatal.2c05492